WRITTEN BY
Neil H Riordan, Luis Gerardo Jiménez Arias and Ramón Coronado
Submitted: September 9th, 2022 Reviewed: October 11th, 2022 Published: October 31st, 2022
DOI: 10.5772/intechopen.108541
Abstract
Numerous and diverse participants are involved in the development of novel therapies: patients, physicians, scientists, sponsors, governing bodies, lawmakers, institutional review boards, and bioethics proponents. While the welfare of the patient must always and unquestionably be at the forefront of any intervention along with informed consent, their wishes, their requests, and their expectations should also be considered at every step. The availability of stem cell research in various countries with dissimilar regulatory agencies has opened the door for thought-provoking questions about their validity from an ethical, legal, and moral perspective, which will be addressed in this chapter, framed within the doctor-patient relationship.
1. Introduction
In 2010, the Ministry of Health of Costa Rica disallowed stem cell therapy, citing concerns about the experimental nature of these procedures. This abrupt decision left several patients unable to continue receiving treatment at the San José hospital – most notably, a young pilot suffering from spinal cord injury after a 2008 airplane accident that rendered him paraplegic with no perspective of ever regaining mobility, according to two independent physicians. Having experienced encouraging progress in muscle recovery and bowel and sexual function following stem cell therapy, [1] he promptly filed a legal remedy for protecting his constitutional rights to the Supreme Court of Costa Rica, along with other patients in a similar predicament. In their appeal, they argued that patients had “a right to exhaust all technically feasible procedures to recover [their] health and quality of life”, citing Article XI of the 1948 American Declaration of the Rights and Duties of Man (the right to the preservation of health and well-being) [2]. This right should not, they contended, be limited by a political authority. Additionally, they invoked the American Convention on Human Rights, namely Articles 5 (the right to physical integrity) and 2 (whereby States Parties undertake to adopt (…) legislative or other measures to grant the rights or freedoms enshrined in The Convention). Lastly, none of the plaintiffs declared having experienced any adverse effects from their therapy at the time of the appeal; they had, in fact, perceived improvements in their health condition.
The Supreme Court ruled that medical treatments had to be permitted by law before their implementation, as had been argued by the defendant (the Ministry of Health). While the judges acknowledged the principle of patient autonomy, the doctor/patient relationship, human dignity, and the right to health, selecting a treatment should rely on the law. However, the Court also recognized that no adverse events had been noted throughout these treatment cycles with stem cells and that they had no factual or legal arguments to halt the treatments that had already begun. The plaintiffs were therefore permitted to continue their therapy.
Six years later, presidential decree n°39,986-S authorized regenerative therapies with adult stem cells in Costa Rica, based on national scientific recommendations, under the human right to health access principles, and citing decades of past research on the safety of hematopoietic stem cells transplants [3]. Therein are outlined the requirements to be submitted by those seeking stem cell therapy administration: safety profile, scientific rationale, cellular characterization, administration, and requirements for the qualifications of health professionals and facilities. While the decree makes a distinction between minimally manipulated and more than minimally manipulated cells, both are permitted with different avenues for authorization.
This Costa Rican case is pivotal because the Supreme Court ruling marks the first legal precedent in a Latin American country that allows patients who are already being treated with stem cells to continue their treatment – a first step, perhaps, to implement international legislation on stem cells. Following the law to the letter in that instance proved to be rather impractical, falling almost into irrationality as it limited the right of access to health for patients who required treatment as a last resort, and going against international law, which stipulated that Costa Rica must have taken the necessary legislative, economic and political measures to facilitate access to the said right to health. The subsequent presidential decree is also a landmark case since it authorizes using a non-minimally manipulated therapeutical product currently not permitted in other jurisdictions, notably in the USA according to the Federal Drugs Administration (FDA) guidance. The compassionate use of or access to drugs and new therapies remains limited on the grounds of minimizing harm to terminally ill patients [4]. How much evidence is necessary to release a given drug or a given experimental therapy? Are Phase I studies sufficient? Can effectiveness be shown at this stage? Answers remain unclear [4].
This chapter will examine legal and moral issues arising from stem cell treatments, the patient/doctor relationship, and the right of access to health and patient welfare in the context of current international regulations and medical tourism – and how conflicting regulations between countries pose conflicting views on ethics regarding patient access to new therapeutics.
2. Stem cells: definitions, applications, and considerations
Human “stem cells” is a broad term that may refer to various types of cells differing in their origin, applications, and characteristics. Hematopoietic stem cells, multipotent, self-renewing, and capable of generating blood cells, were first described in the early 60s [5]. Soon after, another type of multipotent stem cells differing from those of hematopoietic lineages were observed in bone marrow [6] – these would later become known as mesenchymal stem cells [7] or mesenchymal stromal cells (MSCs), with a limited capacity to differentiate into specific types of adult cells. Pluripotent embryonic stem cells (ESCs), capable of giving rise to all cells in the body, were derived in the late 90s from blastocysts [8]. Finally, induced pluripotent stem cells (iPSCs) were introduced in 2006: somatic cells reprogrammed to have the embryonic capacity for differentiation [9]. While much of the research in the 20th century was focused on isolating and identifying techniques to culture stem cells, the early 21st century saw a growing number of case reports and clinical trials seeking to establish their therapeutic potential. This line of research was not without challenges or controversy (Table 1).
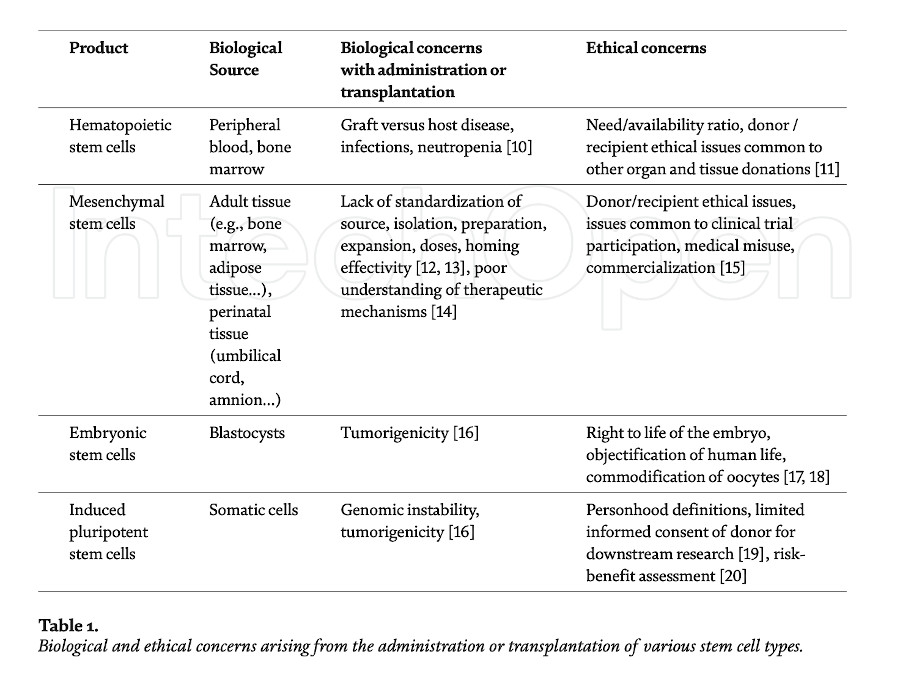
Most of the earlier bioethical controversy was focused, understandably, on the use of embryonic stem cells due to the loss of viable embryos in the process of isolation and derivation of the stem cells, which led to bans or limitations imposed on research in several nations. Mesenchymal stem cells, in contrast, share little of those ethical concerns related to their tissue origin since they can be derived from adult tissue, notably for autologous use, or from perinatal tissue, such as the umbilical cord that would routinely be discarded after a normal birth, for allogeneic use. Consequently, the ethical controversies surrounding the use of mesenchymal stem cells have shifted to considerations applicable to clinical trials and the development and commercialization of novel experimental therapies.
Recent clinical trials with mesenchymal stem cells cover a broad range of tissue sources and administration routes, complicating comparisons between published results. In brief, mesenchymal stem cells appear safe for administration at least in the short term, [21, 22, 23], and some long-term reports are also available [24, 25]. Adverse events are reportedly transient, usually fever or fatigue, and no tumorigenicity or malignancy has been observed thus far [23]. While the mechanisms of action of mesenchymal stem cells are not entirely understood, current research views stem cell secretions as key for their immuno-modulatory, anti-inflammatory, and therapeutic properties. The conditions treated in various clinical trials or case reports include cardiovascular, neurological, autoimmune, orthopedic, pulmonary, and graft-versus-host disease, [26] and more recently, COVID-19 [27]. At least ten products derived from mesenchymal stem cells have received regulatory approval in South Korea, Japan, India, Canada, Australia, and Europe [12]. The wide variety of conditions researched as well as the legitimacy of some products, may give the erroneous impression that mesenchymal stem cells are a “cure-all”: more research is necessary to standardize isolation and culture techniques, as well as to establish risk-benefits and true efficacy for certain conditions to avoid these pitfalls. Most importantly, trials should be conducted ethically, ensuring that disappointing results are also published, that all adverse events are carefully documented and reported, and that patients duly consent with a proper understanding of the procedures and the potential benefits and limits.
Throughout this chapter, “stem cells” should be understood to mean mesenchymal stem cells or adult stem cells unless otherwise indicated.
3. International regulation and medical tourism
The use of stem cells in Costa Rica has been commercially permitted since 2016. The current law does not specify the effectiveness of a treatment or the amounts to be charged, if any. The broad wording was designed to position and cement the place of Costa Rica as a safe biotechnological or medical tourism hub, thereby disregarding ideological debates arising in other countries regarding the restrictions on the use of stem cells. In Turkey, the use of stem cells has been open and regulated as long as they are not embryonic cells, giving way to laws increasingly open to medical tourism [28]. South Korea is a pioneer in the production of stem cell-derived products, [29] notably a composite of allogeneic umbilical cord blood-derived mesenchymal stem cells and hyaluronate to treat cartilage defects in osteoarthritis [24, 30]. In 2014, Japan introduced a “fast-track” regulatory path for regenerative medical products in the Pharmaceutical and Medical Devices Act [31], whereby products can be commercially available following a short trial: patients recruited into that scheme are enrolled in a registry to be followed up for a period during which efficacy must be demonstrated. This law and the subsequent applications of this law were criticized in the international scientific community, [32, 33] using terms such as an “obsession [with staying at the forefront of regenerative medicine]” [34]. Nevertheless, Japan stood its ground [35] and continued research with induced pluripotent stem cells, announcing the start of prospective trials for spinal cord injury and heart conditions in Japanese university hospitals [36, 37], among others. Japanese scientists appear to have followed up on these developments and have published some relevant self-reflection [38, 39, 40].
Irrespective of stem cells, medical tourism is hardly a new phenomenon, and a wealth of literature has been written about ethical considerations and repercussions both in the country of origin and the destination country [41, 42]. Certain countries such as Thailand, Singapore, and India have developed this medical tourism industry to the extent that it represents a non-negligible percentage of their GDP. Contributors to the debate should examine their own biases when referring to foreign countries, avoiding terms such as ‘third world’ or assuming higher possibilities of substandard care, infections, or fraudulence as this may alienate so-called developing nations from much-needed transparency and open cooperation. The perspectives of physicians or researchers from “destination” nations must be included and perhaps should even be the primary source for scientific output, as they should be considered capable of producing critical literature from their own cultural and scientific perspectives. There is a marked inequality in availability and volume of research produced in more affluent countries compared to underprivileged ones, [43] though a process of “catching up” appears to be in place [44].
Nevertheless, medical tourism is a cause of concern, [45] particularly in the case of stem cell therapy, since prospective patients may not be sufficiently informed about the risk and benefits of the treatments they are receiving, or may be charged disproportionate amounts for dubious or unregulated procedures – a form of preying on their state of mind due to their illnesses. Many patients who travel abroad to be treated with stem cells do so as a last resort and in some cases desperately seek a cure or at least an improvement for their medical condition. However, medical malpractice or dishonesty can occur regardless of whether a country approves a given drug or procedure. While medical tourism may contribute or aggravate the proliferation of questionable practices, it can hardly be considered the only reason. Every country has an obligation to implement and uphold good medical practices promoting principles of medical ethics and the deontological standards of medicine. Moreover, the proliferation of fraudulent clinics in some destination clinics is likely hindering the development of legitimate stem cell research [45]. The case of Stamina in Italy is an example where internal regulations successfully identified a fraudulent clinical practice; this operation needed to be halted because of an individual holding no medical qualifications, poor quality standards for manufacturing and no sound scientific rationale, not because of an inherent evil or penchant for fraudulence in stem cell research.
Should medical tourism then be regulated? There is currently no consensus or homogeneity in criteria among countries on regulatory affairs of therapeutic stem cell treatment. The implantation of international regulations would not be a trivial exercise. The suggestion of a recognized agency such as the FDA or the European Medicines Agency (EMA) granting certificates of approval to international clinics, while desirable in theory, would not be possible in practice as it would interfere with the sovereignty of each territory to regulate and legislate their internal affairs. Similarly, the jurisdiction of national agencies is limited to their borders, and allocating a portion of their budget to oversee practices in other countries would not be economically feasible. Some international networking organizations such as the International Society for Cell and Gene Therapy (ISCT), have recently established working groups to tackle regulatory and ethical issues with committee members primarily based in North America, [46] but unlike charters of international intergovernmental organizations, binding for the undersigning nations, their advisories are ultimately non-binding. The Pan American Health Organization (PAHO) has issued recommendations for the use of “advanced therapy medicinal products”, [47] acknowledging that regulatory bodies are still developing in many countries and stressing that ethical implications must be considered, that treatments must take place in authorized, specialized centers, and that a risk-based approach could be considered for establishing regulations. This document notably states that “continual advances in scientific research generally keep it several steps ahead of regulatory mechanisms, a situation that occurs worldwide,” and calls for open communication between scientists and regulatory bodies [47].
Another possibility to curb medical tourism would be to introduce restrictions into the national health network for those patients who have traveled and received treatment in clinics unrecognized by regulatory bodies of e.g., the USA – such punitive measures might, however, not be conducive to the best interests of the patients. Patients may feel they have to lie or be reluctant to request their physician’s opinion, thereby losing an opportunity for education or more extensive research into the treatments they seek abroad. A more constructive solution would then be to actively educate prospective patients before their decision, ensuring that the cost-benefits of stem cell treatment are carefully considered, including the possibility that effectiveness may be small, limited, or non-existent for their particular pathology. The education of patients is hardly a novel idea [48]. The information should be presented in a clear, easily understandable manner rather than a dry scientific report, as this is likely not the format that prospective patients are used to consuming in an era where multimedia is pervasive. Neither should the tone of the information provided be perceived as scolding or belittling, as this may have the unintended effect of the valuable advice being dismissed altogether to the detriment of the patient; efforts should be focused on empowering the patients to make their own decisions (to “know best” or to “be in the know”) – or any other desirable traits that a sociological study into the characteristics of this population may reveal, along with decision-aid studies. For example, they could be more receptive to someone they perceive as a peer than to a disengaged physician or a removed academic seemingly removed from the daily struggles of their conditions. The same narrative and visual techniques employed to attract patients can also be used to educate. Recent efforts, e.g., by the International Society for Stem Cell Research (ISSCR) [49], remain very text-heavy and potentially unengaging for the general public.
However, if after considering the risk/benefits, the patient still decides to seek treatment abroad, be it a clinical trial or a treatment approved by the destination country’s regulatory bodies, at a cost or not, it then becomes the right of a patient to health and the right to access health, to improve their quality of life – this is, perhaps, far more complex to regulate or legislate in an international context. There is a delicate balance between governmental control (imposition or restriction) of therapeutics and the reach of these controls inflicting upon the freedom of the patients to choose what they (along with their medical doctors) want to pursue for their health, under the sole assumption that the patients are fully informed about the risk and possible benefits associated with the treatment.
4. Let doctors be doctors: the patient-doctor relationship
The 2018 Right to Try Act of the USA creates a legal framework for access to unapproved medical products by patients with life-threatening illnesses who have exhausted all their options and may not participate in a clinical trial, provided said product has completed a Phase I study [50, 51]. Opponents of this law have argued that it creates conditions for physicians to prey on desperate patients by creating false hope, that the burden of treatment costs is shifted to patients and manufacturers, and that existing health disparities may be exacerbated, ultimately leading to greater patient suffering [52, 53]. Additionally, valuable information or data collection about product development or adverse events may be lost due to the lack of FDA oversight [52]. The legislation does not compel a manufacturer to provide access to treatment; some manufacturers may outright refuse, [54] and early reports seem to indicate that drugs are still being requested in greater volume under Expanded Access rather than with the Right to Try provision [55, 56]. This legislation raised much controversy, often politically charged, sparking ethical debates about what it was trying to achieve, how much it would truly help, or how it would be implemented in practice. The ethical problems of stem cell therapy then seem no different from that of any other experimental therapy (such as those for cancer or rare diseases contemplated by this law) where the patient’s autonomy, the cost/benefit of the treatment and any possible abuses or misuses must be weighed in. Advocates of the Right to Try Act emphasized the liberty of patients to choose a treatment and to eliminate bureaucracy; in a similar vein, Texas House Bill 810 (85R) authorized the “provision of certain investigational stem cell treatments” under investigation in clinical trials to patients “with certain severe chronic diseases or terminal illnesses.” [57]. The patients were required to provide written informed consent, and the treatment was to be overseen by an Institutional Review Board (IRB), and administered by a certified physician in a hospital, surgical center, or medical school. The IRBs were to submit annual reports of treatments enacted under this law. “Why can’t someone that is of age have the ability to sign off, so to speak, with regard to a proper medical release on the ability to do something that can make such a dramatic difference in their life, and their lifespan, and their quality of life?” argued the proponents [58]. This would also encourage “medical innovation” [58] in the state of Texas – not unlike the intent of countries who have passed similar laws allowing stem cell research or therapy. These two landmark legislations of the United States exemplify a movement to put the patient and their doctor’s relationship at the forefront, which has not been without controversy [59, 60].
If a therapy exists, every effort should be undertaken to implement a way to access said therapy as a last resort for patients. The requirement of having successfully completed Phase I of scientific research may be a reasonable compromise, as long as the patients are sufficiently and objectively educated about the risks and cost/benefits before reaching a decision. And yet, as the example of Japan has shown, a national law, a patient registry, and clinical trials overseen by universities, have still not been considered enough by the academic community. When is it enough? What and whose criteria drive this quantification? Would the debate not be enriched from the participation of physicians and patients who are, so to speak, in the front lines of the battle? One may argue that a “desperate” patient cannot adequately provide informed consent due to their state of mind, but this is a particularly thorny argument that toes close to discrimination, paternalism, [61] or, to use a more modern term, ableism: is having an illness ever sufficient to render a person incapable of making decisions regarding their own welfare? Is a person’s dignity and mental ability lessened or invalidated when faced with a significant loss of quality of life or an eventual end of life? This decision-making process perhaps belongs more in the sphere of a qualified psychiatrist or therapist for each particular case rather than a broad stroke ruling in an academic setting or legislated by a government body. Broadly qualifying a disadvantaged person (in this case, one with an illness) as intrinsically “vulnerable” or incapable of making informed decisions for themselves may be an insult that reinforces the injustices and stigma that they already face [62]. A decision to use or seek new treatments is not in itself irrational; if prospective patients would be considered competent enough to consent to enter a phase I/II trial in a research setting, why could they not consent in a non-research setting under a physician’s supervision? [63]. And if patients have the right to refuse a treatment, surely from this right to refuse follows a reciprocal right to choose or access an intervention [59, 64, 65]. It is an infringement on the right of patient to procure treatment that they understand would be useful for their condition after being presented with clear and truthful information about said treatment. Instead of paternalistic protection, prospective patients need empowerment: a greater voice in setting research agendas and designing studies [62].
Perhaps the most common concern is the potential harm of stem cells themselves or the problems of their commercialization, as the non-maleficence principle must always be kept in mind. The relative safety of mesenchymal stem cells has been sufficiently covered, as reported by systematic reviews [21, 22, 23]. Still, more research is needed for standardization of dosage, culture methods, and source of the stem cells, as well as a need for quantifying effectiveness for clearly defined conditions and thorough documenting of adverse events. Regarding commercialization, as long as there is a demand, there will always be a market to fulfill that demand with various degrees of ethical and legal shades. Stem cell therapy is no stranger to such a conundrum in the face of market greed. A problem of commercialization may derive from health providers being unwilling to fully inform patients about the risk/benefits for fear of losing business; however, not all practices are the same, comparable to medical practices running legally in which their marketing strategies might present skewed information to capture more clients. Within legal boundaries, there could still be unethical behaviors and vice-versa. On the other hand, patients who have gone to great lengths to receive treatment may experience a placebo effect or convince themselves it was worthwhile. Yet incurring in costs to access a treatment is not inherently unethical or fraudulent; the FDA has published a guidance outlining requirements where this practice may be authorized, notably when the costs would be extraordinary to the sponsor because of “manufacturing complexity, scarcity of a natural resource, the large quantity of the drug needed (e.g., based on the size or duration of the trial), or some combination of these or other extraordinary circumstances (e.g., resources available to a sponsor)” [66].
The pressure to find new therapies for illnesses with limited, insufficient, or no current treatment options comes precisely from the physicians, the scientific and medical industry, and patients seeking relief for their conditions. Herein lies the more significant risk: a race for supply and greed of demand when faced with pain or eventual death. Suppose a particular country’s laws or guidelines are restrictive enough to hinder the physician/patient relationship. In that case, doctors may find it impossible to consider alternative therapies, even under compassionate use, due to the lack of adequate protocols. At the same time, patients feel powerless in the face of government regulations. It is precisely at this point where, in desperation, abuses or misuses arise, not from the new therapies themselves, but from a lack of expectations or incomplete information about the possibility of a cure or relief. And one may ask: what’s the rush? Why do some patients insist on seeking a treatment that is not readily available or approved instead of waiting for the due process of clinical research? “The rush is the daily necessity to help sick people. (…) The ‘rush’ arises from our human compassion for our fellow man who needs immediate help,” as “their illnesses will not wait for a more convenient time” [67].
If a legally qualified doctor in his professional authority, after having read results of recent advances in the field, well within the boundaries of the regulatory bodies of the country where they operate concludes that such treatment could help a particular patient, a patient who is willing and fully informed to the best of the current understanding – would they be morally justified in refusing said treatment? Can moral objections ever be a sufficient basis for denying the right to healthcare? Patients who seek stem cell treatments may have spiritual distress or therapeutic hope, [68] an aspect some medical doctors may not be equipped to manage. But what of compassion? This use of “compassionate” may be close to that employed by ecclesiastical authorities who do not oppose but promote treatments that offer at least a better quality of life: a physician’s duty is not limited to knowledge and technical expertise, but also compassion [69]. The compassionate act and the treatment as compassion, coming from the good judgment of a doctor seeking the best for his patient, must therefore be left in that sphere of the doctor’s relationship with his patient. Depersonalized rulings do not allow the physician to exercise his art, profession, and oath. The relationship between the physician, or a team of physicians, and their patients is thus an essential aspect of making an informed decision: one the one hand, the patient exercising his autonomy to make medical decisions, and on the other, the physician, upholding his medical oath and not creating false expectations by promising more than what is expected to be achieved with a given treatment or to create hopes beyond what can be offered. Thus, the final decision to access an intervention must lie in the hands of the patient and their physician, based on real world evidence for safety, and within a sound legal and ethical framework.
“Ethics in both research and clinical settings is most effective when it is preventive” [70]: indeed, bioethicists do not go ahead with scientific developments but discuss scientific issues that are already on the table. Conversely, neither should physicians regard ethical questions as “removed from their daily work at bench or bedside,” as the purpose of new treatments is a societal benefit [70]. “Market will efficiently allocate the resources, but not always in an ethical manner” concerning medical tourism; [41] ethical considerations should therefore be contemplated before the application of treatments by creating a legal framework that promotes scientific research and keeps the welfare of the patient at the forefront. The fear of possible misuse or medical misconduct should not deny the patients’ right to health, particularly when their lives are at risk or when they are the most vulnerable to their condition. Accumulated clinical experience and evidence-based medicine about safety, dosage, and efficacy would be more appropriate when determining whether to offer or withhold access to treatment.
5. Legal and moral issues
The international legal system is derived from ethical principles, at the heart of which lies the dignity of the human person, and modern bio-law too draws from ethical and axiological foundations. Human dignity as a concept is fundamentally imprecise, as its definition necessitates defining first what is dignified or worthy, and what is unworthy – an anthropocentric, Judeo-Christian notion [71]. Subsequently, the western concept of human dignity has evolved and dissociated itself from any deity to accept the teleological interpretation of the end of man (Kant): each person is an end in itself and not a means to satisfy the end of another person. In any case, a more pertinent application would be establishing what makes an act respectful of the dignity of others. A philosophical question arises as to whether human dignity is opening up in the last century, whether it is facing threats as never before, or whether both are occurring simultaneously [72]. But if dignity is being threatened, it is first necessary to seek and recognize, that is to say, to pinpoint these threats. One of the possible threats to human dignity comes precisely from biotechnological development and the interference of politicized legal systems that could be restricting fundamental rights, such as the right to health and the right to life. International treaties regarding human dignity appear to have different intensities and interpretations when applied to concrete cases [73], in legal cases ranging from political disappearances to in-vitro fertilization [74]. Interestingly, in the latter, a court of law and not science has defined a biological fact. Are we then facing an ideological system of human rights? Do human rights serve to protect life and health? Are all lives of equal value or are some worth less than others? Are scientific truths at the service of the law or should the law adjust to the reality of biotechnological progress?
The Catholic Church has been a notable exponent of the ethics of stem cell research and therapy, with contributions from the various Congregations of the Holy See and various speeches and interviews of the Popes after the Second Vatican Council promoting scientific development hand in hand with ethics. “Progress becomes true progress only if it serves the human person and if the human person grows: not only in terms of his or her technical power, but also in his or her moral awareness,” Pope Benedict XVI declared in 2006. Science shows its usefulness most strongly and richly when its end is to alleviate human suffering through new findings and resources: the efforts of the researchers result in the improvement of the affected and the different conditions or diseases. Stem cell research was deemed deserving of encouragement when it combined scientific knowledge, the most advanced technology in the biological field, and the ethics advocating respect for the human being in all phases of his existence [75]. Man is the actor of scientific research, but he is often the object of this same research; he must consequently be the beneficiary of scientific research, but never a mere instrument. Man cannot be disposed of as an object of research or commercialization, especially when his state is more vulnerable, in accordance with all the principles of personalist ethics; the main interest is the well-being of all and, in particular, of each individual [76]. Research initiatives with adult stem cells were deemed to be free of ethical problems, and clinical use presented no moral objections as long as “scientific rigor and prudence [reduced] any risks to the patient to the bare minimum and [facilitated] the interchange of information among clinicians and full disclosure to the public at large” [77]. A repeated call is thus made for dialog between science and ethics, particularly when the fruits of research remain inaccessible to those who lack the means to access them. “Advances in medical science,” it was noted, “go hand in hand with just and equitable provision of health-care services,” [75] and one may add to this, with public health policies that are willing and open to enable access to those fruits of research. The path of advancement thus leads to the promotion structures and economic means conducive to scientific achievements.
The morality of medical tourism from the perspective of prospective patients has been examined before, considering whether it is moral to “jump the queue” in countries with socialized medicine to seek care in countries where ordinary citizens may not afford the facilities offered to medical tourists. While some patients understood the perspective of the greater good, they were more willing to solve their problems (pain) than to consider fairness or morality at the time of their decision [78]. At the heart of this conflict lie two divergent ethical frameworks: the rights-based and the communitarian frameworks. In rights-based approach, “the rights and dignity of the individual should never (or rarely) be sacrificed to the interests of the larger society”, whereas the “common good” will be at the center of communitarian views, where policies will be shaped to promote these “shared values, ideals and goals of a community” [64]. Having a thorough regulatory process protects the needs of the community, or more precisely, of the future members of the community who will become sick – paradoxically to the detriment of those who are currently ill or suffering and consequently have good reasons to prefer a quicker process or an alternative approach [59]. To what extent can the goal of seeking relief be considered immoral? When a patient fully and objectively informed decides to try an experimental or unproven therapy in a country where it is regulated, within any means reasonably available to them, should their freedom to choose be curtailed? Is there a price to feeling well, to regain mobility? To life?
The case of Ashya King, [79, 80] a young patient from the UK suffering from a brain tumor in 2015, is a dramatic example of the difficult decisions patients and their families encounter when at odds with current regulations or laws. Unwilling to subject him to radiation and chemotherapy at such a young age, the parents expressed a desire to try proton beam therapy in Prague, which was at that time not approved in the UK. When they were denied this option, the parents signed the child out of the hospital and attempted to travel to Prague – but an arrest warrant was issued against them because of potential child endangerment. After a short judicial deliberation, they were able to reach Prague. The intervention was successful; as of 2018, the child was reportedly in good health with cancer in remission [81]. The UK approved proton beam therapy shortly after positive results from a clinical trial, as it was safe and had fewer side effects than chemotherapy [82]. Randomized trials comparing proton therapy (desired by the parents) and traditional radiation (proposed by the UK health system) were deemed to be “unethical and not feasible” [82] and this was “likely to be the best evidence available” [83]. Clinical trials are the gold standard of research, but of what good would that have been to the child in 2015? He did not have the time to wait for this approval as he needed treatment as a matter of life or death. Was it morally justified to withhold this treatment option from him? Was it fair to arrest parents for seeking treatment, unapproved in their country, that ultimately would save their child’s life and preserve his quality of life? The intervention was successful, but even if it had not been, was it immoral for them to try? Those who are healthy and not living with a significant disability should, perhaps, strive to achieve a greater understanding of the mindset and the goals of someone who is in pain or significantly impaired before they cast moral judgments on their actions, or seek to limit their liberties as self-appointed moral arbitrators. In the late 80s, when genetic therapy was in its infancy, the parent of three sick children wrote in the context of sickle cell anemia research, “I resent the fact that a few well-meaning individuals have presented arguments strong enough to curtail the scientific technology which promises to give some hope. Aren’t they deciding what’s best for me without any knowledge of my suffering?” [84]. Prospective patients may feel similarly bewildered when being deprived of their autonomy and their capacity to make their own decisions, and so the detached intellectual debate should be balanced with compassion [67] and with respect for the self-determination of patients.
6. Future perspectives
The case of Japan merits more consideration rather than outright dismissal. Following a desire to regulate the proliferation of stem cell clinics with dubious practices, a law was passed after various committee reports, some including medical professionals, lawyers, and lay participants [38]. The development of this legislative action must be understood in the Japanese context, which recognizes the right to access to health care and protects the freedom of discretion and academic freedom (physician’s discretion) [38]. Subsequently, pertinent arrests have been made in cases where cells were processed without proper regulatory authorization [38, 85]. The approval of one ophthalmological trial with induced pluripotent stem cells was subject to a committee review process, where risks to patients were examined, along with how information was to be presented to prospective patients (who additionally had significant visual impairments) for informed consent [40]. The process in the latter was not perfect, [40] and neither is the current law [38]. Still, as Japanese researchers note, there is potential for suggestions, amendments, and recommendations to strengthen the practice of regenerative medicine in Japan: “If various case studies on the review processes of [stem cell trials] or other cutting-edge biotherapeutic trials from around the world were similarly discussed, such case studies would contribute to establishing or improving guidelines for review committees to further improve the quality of these discussions” [40]. That is, perhaps, the most tragic aspect of the case of Japan: a country exercising its national sovereignty, enacted legislation with reasonable regulatory controls, but it was maligned by academics in other countries since its inception. Yet Japanese researchers have demonstrated they are capable of internal criticism and suggestions for improvement. Rather than jumping to conclusions of fraud, obsessions, or lack of oversight, countries should be encouraged and trusted to develop their regulatory laws with guidance from recognized international health bodies, and scientists of said countries can be held accountable for upholding ethical, medical, and scientific standards.
Some notable lessons may be learned from the history of excimer laser. More commonly known as “LASIK”, it was developed in the 1970s and applied to ophthalmology in the late 80s. Compared to other countries, the FDA was notoriously slow to authorize this intervention, or more precisely, the manufacturing process for the laser technology. During the lengthy review process, interesting questions such as “what complication rates are acceptable?”; “what length of follow-up is acceptable [for complications]?”; “what is an excellent study design for a clinical trial (…)?”; “how good must a surgical procedure (…) be before it will be made widely available for clinical use in this country?” [86]. These questions have many similarities with the current debates regarding stem cell trials. Excimer laser investigators “expressed frustration with the deliberate pace of the review process, while outside the United States large numbers of patients [were] having the surgery” in Canada, Europe, Australia, and Asia [86]. Indeed, clinics from those countries established “systems of referral in which Americans fly in for surgery and return home for post-operative care” [86]. The FDA approved the procedure between 1995 and 1999 following the usual clinical trial phase format. Excimer laser surgeries were extremely popular in the 2000s in the USA, then declined notably, [87] with a resurgence during the pandemic presumably due to mask wearing. The reasons for this decline in popularity are not well understood, but some patients cite concerns about complications after reading about the experiences of other patients [88]. There are lessons to be learned here: patients WILL find a way within their means to pursue a novel treatment or procedure they believe will help them, particularly if regulatory bodies are slow on the uptake. The conclusion is not necessarily that regulatory bodies should be less careful or quicker, but given that patients will seek treatment where it is available, once again education and information are of utmost importance. Indeed, once more information about excimer laser became available, even with the low rate of complications, some patients decided on their own that the procedure was not worth it or that they were unwilling to risk it. Thus, patients too should be trusted in their capability to exercise their own educated decisions when it comes to their access to health provided that they are given sufficient guidance.
The development, introduction and “fine-tuning” of stem cell interventions are perhaps not so different from the history of other medical breakthroughs, but this debate has become peculiarly heated, sometimes emotional. All stakeholders, patients, patient advocates, scientists, regulators, and clinicians, even those currently offering unproven therapies (as not all are fraudulent or untrustworthy, and may desire fair regulation) must engage in a constructive dialog to define acceptable policies [89]. This dialog would ideally lead to humane, ethical, scientifically sound, and commercially viable regulations, as long as every party listens actively and does not react with frustration when presented with differing points of views. The Colombian Xaverian university recently collaborated with multiple countries to develop an ISO for “biobanking of human mesenchymal stromal cells derived from bone marrow,” [90] with debates and meetings in Berlin, Tokyo and Toronto, [91] proving cross-border collaboration is possible to develop international standards for stem cells. We are past the time of pointing fingers or engaging in unproductive academic accusations: all stakeholders must arrive at a consensus with the ultimate goal of developing sound regulatory policies at the international and national level, from which the rest will derive: non-fraudulent, ethically-driven clinics, physicians exercising their medical criteria, and patients empowered to make their own informed decisions.
Acknowledgments
The authors would like to thank Ms. Dorita Avila for manuscript preparation for publication.
Conflict of interest
NHR is a shareholder of Medistem Panama. RC and LGJA declare no conflicts of interests.
References
1. Ichim TE, Solano F, Lara F, Paris E, Ugalde F, Rodriguez JP, et al. Feasibility of combination allogeneic stem cell therapy for spinal cord injury: A case report. International Archives of Medicine. 2010;3:30
2. Organization of American States, editor. American Declaration of the Rights and Duties of Man. Bogotá: Ninth International Conference of American States; 1948
3. Executive Power of Costa Rica. Decreto Ejecutivo N° 39986-S: Autorización para las Terapias Regenerativas con Células Madre Adultas. San José, Costa Rica: La Gaceta Costa Rica; 2016
4. Falit BP, Gross CP. Access to experimental drugs for terminally ill patients. Journal of the American Medical Association. 2008;300(23):2793-2795
5. Till JE, McCulloch EA. A direct measurement of the radiation sensitivity of normal mouse bone marrow cells. Radiation Research. 1961;14(2):213-222
6. Friedenstein AJ, Petrakova KV, Kurolesova AI, Frolova GP. Heterotopic transplants of bone marrow. Transplantation. 1968;6(2):230-247
7. Caplan AI. Mesenchymal stem cells. Journal of Orthopaedic Research. 1991;9(5):641-650
8. Thomson JA, Itskovitz-Eldor J, Shapiro SS, Waknitz MA, Swiergiel JJ, Marshall VS, et al. Embryonic stem cell lines derived from human blastocysts. Science. 1998;282(5391):1145-1147
9. Takahashi K, Yamanaka S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell. 2006;126(4):663-676
10. Smith LA, Wright-Kanuth MS. Complications and risks in hematopoietic stem cell transplant patients. Clinical Laboratory Science. 2001;14(2):118-124
11. Liso A, Neri M, Maglietta F, La Russa R, Turillazzi E. Hematopoietic stem cell transplantation: A bioethical lens. Stem Cells International. 2017;2017:1286246
12. Levy O, Kuai R, Siren EMJ, Bhere D, Milton Y, Nissar N, et al. Shattering barriers toward clinically meaningful MSC therapies. Science Advances. 2020;6(30):eaba6884
13. Regmi S, Pathak S, Kim JO, Yong CS, Jeong JH. Mesenchymal stem cell therapy for the treatment of inflammatory diseases: Challenges, opportunities, and future perspectives. European Journal of Cell Biology. 2019;98(5-8):151041
14. Gao F, Chiu SM, Motan DA, Zhang Z, Chen L, Ji HL, et al. Mesenchymal stem cells and immunomodulation: Current status and future prospects. Cell Death & Disease. 2016;7:e2062
15. MacPherson A, Kimmelman J. Ethical development of stem-cell-based interventions. Nature Medicine. 2019;25(7):1037-1044
16. Suman S, Domingues A, Ratajczak J, Ratajczak MZ. Potential clinical applications of stem cells in regenerative medicine. Advances in Experimental Medicine and Biology. 2019;1201:1-22
17. de Miguel-Beriain I. The ethics of stem cells revisited. Advanced Drug Delivery Reviews. 2015;82-83:176-180
18. Holland S, Lebacqz K, Zoloth L. The Human Embryonic Stem Cell Debate: Science, Ethics, And Public Policy. Cambridge, Massachusetts, USA: MIT Press; 2001. ISBN: 9780262082990
19. Zheng YL. Some ethical concerns about human induced pluripotent stem cells. Science and Engineering Ethics. 2016;22(5):1277-1284
20. Fung RK, Kerridge IH. Uncertain translation, uncertain benefit and uncertain risk: Ethical challenges facing first-in-human trials of induced pluripotent stem (ips) cells. Bioethics. 2013;27(2):89-96
21. Lalu MM, McIntyre L, Pugliese C, Fergusson D, Winston BW, Marshall JC, et al. Safety of cell therapy with mesenchymal stromal cells (SafeCell): A systematic review and meta-analysis of clinical trials. PLoS One. 2012;7(10):e47559
22. Thompson M, Mei SHJ, Wolfe D, Champagne J, Fergusson D, Stewart DJ, et al. Cell therapy with intravascular administration of mesenchymal stromal cells continues to appear safe: An updated systematic review and meta-analysis. EClinicalMedicine. 2020;19:100249
23. Wang Y, Yi H, Song Y. The safety of MSC therapy over the past 15 years: A meta-analysis. Stem Cell Research & Therapy. 2021;12(1):545
24. Park YB, Ha CW, Lee CH, Yoon YC, Park YG. Cartilage regeneration in osteoarthritic patients by a composite of allogeneic umbilical cord blood-derived mesenchymal stem cells and hyaluronate hydrogel: Results from a clinical trial for safety and proof-of-concept with 7 years of extended follow-up. Stem Cells Translational Medicine. 2017;6(2):613-621
25. Petrou P, Kassis I, Ginzberg A, Halimi M, Yaghmour N, Abramsky O, et al. Long-term clinical and immunological effects of repeated mesenchymal stem cell injections in patients with progressive forms of multiple sclerosis. Frontiers in Neurology. 2021;12:639315
26. Galderisi U, Peluso G, Di Bernardo G. Clinical trials based on mesenchymal stromal cells are exponentially increasing: Where are we in recent years? Stem Cell Reviews and Reports. 2022;18(1):23-36
27. Lanzoni G, Linetsky E, Correa D, Messinger Cayetano S, Alvarez RA, Kouroupis D, et al. Umbilical cord mesenchymal stem cells for COVID-19 acute respiratory distress syndrome: A double-blind, phase 1/2a, randomized controlled trial. Stem Cells Translational Medicine. 2021;10(5):660-673
28. Ozturk Turkmen H, Arda B. Ethical and legal aspects of stem cell practices in Turkey: Where are we? Journal of Medical Ethics. 2008;34(12):833-837
29. Lee S, Kwon T, Chung EK, Lee JW. The market trend analysis and prospects of scaffolds for stem cells. Biomaterials Research. 2014;18:11
30. Lim HC, Park YB, Ha CW, Cole BJ, Lee BK, Jeong HJ, et al. Allogeneic umbilical cord blood-derived mesenchymal stem cell implantation versus microfracture for large, full-thickness cartilage defects in older patients: A multicenter randomized clinical trial and extended 5-year clinical follow-up. Orthopaedic Journal of Sports Medicine. 2021;9(1):2325967120973052
31. Japanese Law Translation Database System (Ministry of Justice of Japan). Act on Securing Quality, Efficacy and Safety of Products Including Pharmaceuticals and Medical Devices. Available from: https://www.japaneselawtranslation.go.jp/en/laws/view/3213#je_ch6
32. Cyranoski D. Japan’s approval of stem-cell treatment for spinal-cord injury concerns scientists. Nature. 2019;565(7741):544-545
33. Editorial. A stem-cell race that no one wins. Nature. 2019;573(7775):463
34. Editorial. Stem the tide. Nature. 2015;528(7581):163-164
35. Miyamoto S. Japan responds: Stem-cell therapy justified. Nature. 2019;569(7754):40
36. Keio University School of Medicine. Clinical Research on First-In-Human Clinical Trial of Transplantation: Regenerative Medicine Using iPS Cell-Derived Neural Stem/Progenitor Cells to Treat Complete Subacute Spinal Cord Injury. Tokyo, Japan: Keio University Hospital Press Release; 2022. Available from: https://www.keio.ac.jp/en/press-releases/2022/Jan/14/49-92092/
37. Graduate School of Medicine. Doctor-Initiated Clinical Trial Begun for Cell Spray Heart Failure Treatment Method. Suita, Japan: Osaka University Hospital Press Release; 2019. Available from: https://www.med.osaka-u.ac.jp/eng/archives/5618
38. Takashima K, Morrison M, Minari J. Reflection on the enactment and impact of safety laws for regenerative medicine in Japan. Stem Cell Reports. 2021;16(6):1425-1434
39. Matsushita S, Tachibana K, Kusakabe T, Hirayama R, Tsutsumi Y, Kondoh M. The roadmap to approval under Japan’s two-track regulatory system: Comparing six regenerative medical products. Cell Stem Cell. 2020;27(4):515-518
40. Takashima K, Inoue Y, Tashiro S, Muto K. Lessons for reviewing clinical trials using induced pluripotent stem cells: Examining the case of a first-in-human trial for age-related macular degeneration. Regenerative Medicine. 2018;13(2):123-128
41. Badulescu D, Badulescu A. Medical tourism: Between entrepreneurship opportunities and bioethics boundaries: Narrative review article. Iranian Journal of Public Health. 2014;43(4):406-415
42. NaRanong A, NaRanong V. The effects of medical tourism: Thailand’s experience. Bulletin of the World Health Organization. 2011;89(5):336-344
43. Harzing A-W, Giroud A. The competitive advantage of nations: An application to academia. Journal of Informetrics. 2014;8(1):29-42
44. Radosevic S, Yoruk E. Are there global shifts in the world science base? Analysing the catching up and falling behind of world regions. Scientometrics. 2014;101(3):1897-1924
45. Lyons S, Salgaonkar S, Flaherty GT. International stem cell tourism: A critical literature review and evidence-based recommendations. International Health. 2022;14(2):132-141
46. ISCT Head Office. ISCT Establishes Working Group to Tackle Regulatory and Ethical Issues with Expanded Access. Vancouver, Canada: Telegraft Hub. ISCT News and Community; 2022. Available from: https://www.isctglobal.org/telegrafthub/blogs/isct-head-office1/2022/02/28/eaworkinggroup
47. Pan American Health Organization. Regulation of advanced therapy medicinal products: Concept note and recommendations. In: Ninth Conference of the Pan American Network for Drug Regulatory Harmonization (PANDRH). San Salvador, October 2018: PAHO/HSS/19-004; 2019
48. Master Z, Robertson K, Frederick D, Rachul C, Caulfield T. Stem cell tourism and public education: The missing elements. Cell Stem Cell. 2014;15(3):267-270
49. International Society for Stem Cell Research. A Closer Look at Stem Cells. Available from: https://www.closerlookatstemcells.org/
50. 115th Congress (2017-2018). H.R.878 – 115th Congress (2017-2018): Right to Try Act of 2017. 2017. Available from: https://wwwcongressgov/bill/115th-congress/house-bill/878
51. Agarwal R, Saltz LB. Understanding the right to try act. Clinical Cancer Research. 2020;26(2):340-343
52. Coughlin C, King NMP, McKinney M. Regenerative medicine and the right to try. Wake Forest Journal of Bus & Intell Prop L. 2017;18:590
53. Mahant V. “right-to-try” experimental drugs: An overview. Journal of Translational Medicine. 2020;18(1):253
54. Cohen T. Exclusive: BrainStorm will not provide ALS therapy under U.S. right to try act. Reuters. Healthcare & Pharma. 2018. Available from: https://www.reuters.com/article/us-health-brainstorm-cell-als-exclusive/exclusive-brainstorm-will-not-provide-als-therapy-under-u-s-right-to-try-act-idUSKBN1JM1BE
55. Zettler ME, Jeune-Smith Y, Feinberg BA, Phillips EG Jr, Gajra A. Expanded access and right to try requests: The community Oncologist’s experience. JCO Oncology Practice. 2021;17(11):e1719-e1e27
56. Mehzer M. GAO Reports on FDA, Drugmaker Efforts to Boost Access to Investigational Drugs. www.raps.org
57. Texas Legislative Session 85(R). HB 810. Relating to the Provision of Certain Investigational Stem Cell Treatments to Patients with Certain Severe Chronic Diseases or Terminal Illnesses and Regulating the Possession, Use, and Transfer of Adult Stem Cells; Creating a Criminal Offense. 2017. Available from: https://capitoltexasgov/billlookup/Historyaspx?LegSess=85R&Bill=HB810
58. Matthews K, Kunisetty B, Sprung K, Texas HB. 810: Increased access to stem cell interventions or an increase in unproven treatments? Stem Cells and Development. 2018;27(21):1463-1465
59. Brodrick M. Free to choose: A moral defense of the right-to-try movement. The Journal of Medicine and Philosophy. 2020;45(1):61-85
60. Servick K. Texas signals support for unproven stem cell therapies. Science. 2017;356(6344):1219
61. Woods S, McCormack P. Disputing the ethics of research: The challenge from bioethics and patient activism to the interpretation of the declaration of Helsinki in clinical trials. Bioethics. 2013;27(5):243-250
62. Debruin D. Reflections on “vulnerability”. Bioethics Examiner. 2001;5(2):1-4
63. Robertson JA. Controversial medical treatment and the right to health care. The Hastings Center Report. 2006;36(6):15-20
64. Chahal M. Off-trial access to experimental cancer agents for the terminally ill: Balancing the needs of individuals and society. Journal of Medical Ethics. 2010;36(6):367-370
65. Brodrick M. Evolving Ethics of ‘Right to Try’ Unproven Drugs. Morrisville, North Carolina, USA. Medical Ethics Advisor: Relias Media; 2021. pp. 13-24
66.
United States Food and Drug Administration. Charging for Investigational Drugs Under an IND – Questions and Answers. 2016. Available from: https://www.fda.gov/regulatory-information/search-fda-guidance-documents/charging-investigational-drugs-under-ind-questions-and-answers
67. Anderson WF. What’s the rush? Human Gene Therapy. 1990;1(2):109-110
68. Hyun I. Therapeutic hope, spiritual distress, and the problem of stem cell tourism. Cell Stem Cell. 2013;12(5):505-507
69. Pope Francis. To Directors of the Orders of Physicians of Spain and Latin America. 2016. Available from: https://wwwvaticanva/content/francesco/en/speeches/2016/june/documents/papa-francesco_20160609_ordini-medici-spagna-america-latinahtml
70. King NM, Perrin J. Ethical issues in stem cell research and therapy. Stem Cell Research & Therapy. 2014;5(4):85
71. Pele A. Una aproximación al concepto de dignidad humana. Universitas: Revista de filosofía, derecho y política. 2004;1:9-13
72. Spaemann R. Sobre el concepto de dignidad humana. Persona y Derecho. 1988;19:13-33
73. Bohórquez Monsalve V, Aguirre RJ. As tensões da dignidade humana: conceituação e aplicação no direito internacional dos direitos humanos. Sur Revista Internacional de Direitos Humanos. 2009;6(11):40-63
74. Corte Interamericana de Derechos Humanos. Ficha Técnica: Artavia Murillo y otros (Fertilización in Vitro) Vs. Costa Rica Sentencia de excepciones preliminares, fondo, reparaciones y costas N° 257. 2012. Available from: https://www.corteidh.or.cr/cf/Jurisprudencia2/ficha_tecnica.cfm?nId_Ficha=235
75. Pope Benedict XVI. Address of His Holiness Benedict XVI to Participants in the International Conference Promoted by The Pontifical Council for Culture. 2011. Available from: https://wwwvaticanva/content/benedict-xvi/en/speeches/2011/november/documents/hf_ben-xvi_spe_20111112_stem-cellshtml
76. Ratzinger J, Dio E, Mondo I. Essere Cristiani Nel Nuovo Millennio. Revista Agustiniana. 2002;43:43
77. Pope Benedict XVI. Instruction Dignitas Personae on Certain Bioethical Questions. 2008. Available from: https://wwwvaticanva/roman_curia/congregations/cfaith/documents/rc_con_cfaith_doc_20081208_dignitas-personae_enhtml
78. Snyder J, Crooks VA, Johnston R. Perceptions of the ethics of medical tourism: Comparing patient and academic perspectives. Public Health Ethics. 2012;5(1):38-46
79. Keener AB. Arrests reveal debate about costs and benefits of proton therapy. Nature Medicine. 2014;20(10):1081
80. O’Brien A, Sokol DK. Lessons from the Ashya King case. BMJ. 2014;349:g5563
81. Adams J. Ashya King cleared of cancer three years after his parents abducted him from hospital for treatment abroad. The LA Times. The Telegraph. 2018. Available from: https://www.telegraph.co.uk/news/2018/03/03/ashya-king-cleared-cancer-three-years-parents-abducted-hospital/
82. Yock TI, Yeap BY, Ebb DH, Weyman E, Eaton BR, Sherry NA, et al. Long-term toxic effects of proton radiotherapy for paediatric medulloblastoma: A phase 2 single-arm study. The Lancet Oncology. 2016;17(3):287-298
83. NHS. Proton Beam Therapy ‘effective’ and ‘Causes Fewer side Effects’: Defunct Site. 2016. Available from: https://web.archive.org/web/20160201230211/http://www.nhs.uk/news/2016/02February/Pages/Proton-beam-therapy-effective-and-causes-fewer-side-effects.aspx
84. Siegel B. More labs than patients : Desire to Be first colors gene studies. Los Angeles Times. 1987. Available from: https://www.latimes.com/archives/la-xpm-1987-12-14-mn-19227-story.html
85. Sipp D, Okano H. Japan strengthens regenerative medicine oversight. Cell Stem Cell. 2018;22(2):153-156
86. McDonnell PJ. Excimer laser photorefractive keratectomy. The Food and Drug Administration panel speaks. Archives of Ophthalmology. 1995;113(7):858-859
87. Stuart A. A look at LASIK past, present and future. American Academy of Ophthalmology. San Francisco, California, USA: EyeNet Magazine. American Academy of Ophthalmology; June 2009. Available from: https://www.aao.org/eyenet/article/look-at-lasik-past-present-future?june-2009
88. Schoenberg N. Lasik surgery falling out of favor with patients. chicagotribunecom. The Chicago Tribune. 2016. Available from: https://www.chicagotribune.com/lifestyles/health/sc-lasik-loses-luster-health-0525-20160526-story.html
89. Matthews KR, Iltis AS. Unproven stem cell-based interventions and achieving a compromise policy among the multiple stakeholders. BMC Medical Ethics. 2015;16(1):75
90. International Organization for Standardization. ISO 24651:2022. Biotechnology — Biobanking — Requirements for Human Mesenchymal Stromal Cells Derived from Bone Marrow. Geneva, Switzerland: ISO; 2022
91. Martínez DM. Colombiana lidera reglamentación mundial para cierto tipo de células madre. Bogotá, Colombia: Pontificia Universidad Javeriana (a university in Colombia). 2022. Available from: https://www.javeriana.edu.co/pesquisa/celulas-madre-colombiana-reglamenta-investigacion/

